NK cell response to the human polyomavirus JC (JCV)
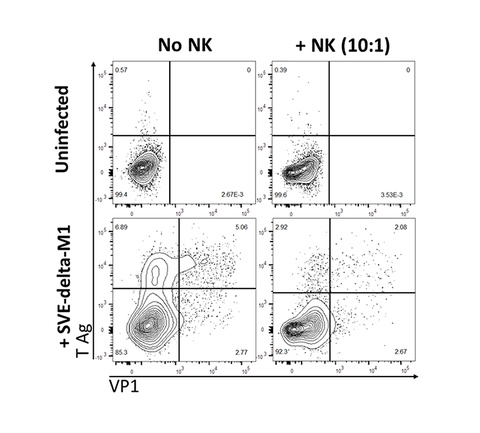
NK cell response to the human polyomavirus JC (JCV)
JCV infects most healthy individuals without causing any disease. In immune compromised patients, JCV can cause the often fatal brain infection – progressive multifocal leukoencephalopathy (PML). There is currently no available treatment for PML. The only option to clinicians is to boost immune response, which is not easily achieved in most cases. We are the first to discover NK cell control of JCV-infected cells, adding another aspect of the immune arsenal to fight JCV. Current studies in lab focus on how to augment and harness NK cells to specifically target JCV.
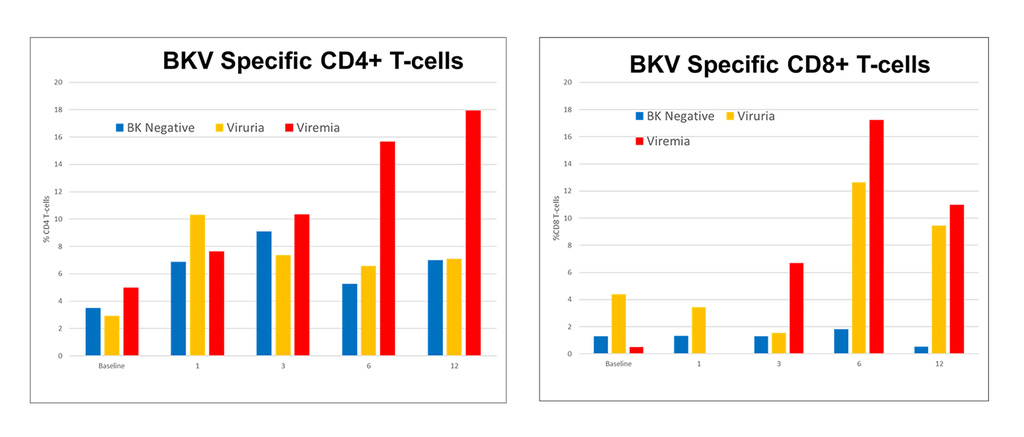
NK cell response to the human polyomavirus BK (BKV)
NK cell response to the human polyomavirus BK (BKV)
Like JCV, BKV also infects most healthy individuals without causing any disease. But in kidney transplant recipients, BKV causes nephropathy and can result in loss of the transplanted graft. There is no treatment for BKV. We are mapping the NK cell response to BKV.
Organoids infection with human polyomaviruses JC and BK– There is no animal model for JCV infection. To improve upon the 2D cell culture model of JCV infection, we are creating human organoids to better study JCV virology and immunology.
Non human primate model of JCV infection
Non human primate model of JCV infection
There is no animal model for JCV infection. In collaboration with University of Wisconsin Primate center, we are infecting rhesus macaques with JCV to create a non human primate model for PML.
SIV neuropathogenesis
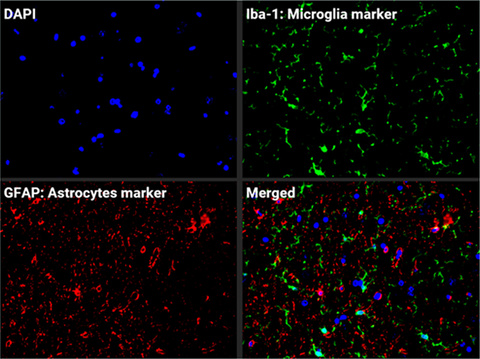
SIV neuropathogenesis
While HIV is now well controlled by antiretroviral therapy in infected individuals, neuro inflammation and cognitive alterations remain an unsolved problem. Using the SIV infection of rhesus macaques, we are dissecting the intricate viral immune and inflammation pathways in the central nervous system to find treatment for patients.
SARS-CoV-2 Neuropathogenesis
SARS-CoV-2 Neuropathogenesis
Using the rhesus macaque model, we are studying the neuro immune and inflammatory response to SARS-CoV02.